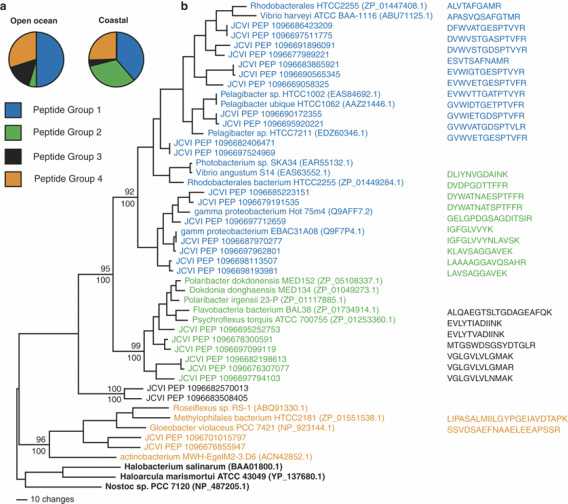
S. Atlantic Ocean Metaproteomics
|
FROM ISME RESEARCH ARTICLE
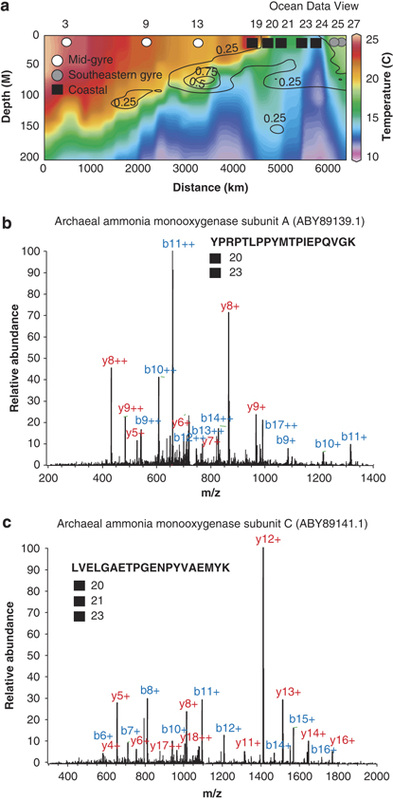
"Archaeal ammonia monooxygenase proteins identified in the Benguela upwelling region. (a) Temperature profile with nitrite overlay (contours) plotted in Ocean Data View (Schlitzer, 2002). Nitrite concentrations are in μm (Table 1). Stations as per Figure 1; ordered here by distance from first sampling following the cruise track. (b) Tandem mass spectrum of a peptide matching candidatus Nitrosopumilus maritimusammonium monooxygenase A (AmoA) and (c) of peptide matching AmoC." "TBDTs accounted for 19% of all tandem mass spectra identified in this study and were identified in every sample. In addition, cytoplasmic transmembrane components of the TonB complex (MotA/TolQ/ExbB) were identified in all coastal samples and in two open ocean samples. TonB complexes are known to transport essential compounds across the outer membrane of Gram-negative bacteria using the cytoplasmic membrane PMF (Nikaido, 2003). This is termed mechanical energy transduction because energy from PMF causes a conformational shift in the structure of TonB. TBDT activities were once thought to be restricted to iron complexes (siderophores) and vitamin B12 (cobalamin). Recent experimental and bioinformatic studies indicate that nickel, cobalt, copper, maltodextrins, sucrose, thiamin and chito-oligosaccharides are also substrates for TBDTs (Schauer et al., 2008). It can be noted that the Bacteroides thetaiotaomicron genome contains over 120 TBDTs, suggesting that the potential range of substrates can be quite broad. At present, we are unable to ascertain the substrate specificity of the distinct TBDT proteins identified here, but their ubiquitous identification in our samples suggests that TBDT activities are an important mechanism for microbial nutrient acquisition in diverse ecosystems across a broad swath of the South Atlantic. Basin-scale metaproteomics revealed ubiquitous TBDT and rhodopsin energy transduction processes. Indeed, in some cases, we infer that both proteins were found in the same taxonomic lineage (Supplementary Figure 4). TBDT transport of nickel, maltodextrin and sucrose does not require additional ABC transporter activities to cross the cytoplasmic membrane, suggesting that the PMF is the sole energy source for TonB-activated transport of some compounds (Neugebauer et al., 2005; Blanvillain et al., 2007; Schauer et al., 2007). These observations, coupled with the lineage-specific geographic patterns of rhodopsin expression seen here, suggest that the light-induced PMF may serve functions other than ATP synthesis (Fuhrman et al., 2008)." |