Red Tide Allelopathy
Marine plankton are responsible for
approximately 50% of global primary production, and control much of the global
carbon, nitrogen and silicon budgets. In order to mediate interactions among
these planktonic organisms, chemical cues and signals are critically important for
competition, defense against grazers, and predator detection. As a result, these chemical cues that affect
ecological interactions between microalgae are hypothesized to alter large
scale ecosystem processes. Although allelopathy, the production of chemicals
released by competing species to inhibit or terminate growth, is well known as
a mechanism used to structure species
succession and community composition, the mechanisms of action of allelopathy
have barely been studied. Karenia brevis,
a red tide forming dinoflagellate, is allelopathic to multiple
competitors, which may allow it to monopolize blooms along U.S. coastlines. The aim of this study was to use an integrated
systems biology approach in order to investigate and define the physiological
impacts of K. brevis on phytoplankton
competitors.
Integrating metabolomic and proteomic analyses, we identified how allelopathy impacts competitor metabolism and what biochemical strategies are undertaken by competitors to adapt to allelopathy. Effects on competitor physiology were reflected in the expressed metabolomes and proteomes of two diatom species, with the diatom that co-occurs with K. brevis blooms (A. glacialis) exhibiting more robust metabolism in response to K. brevis. The greater metabolic sensitivity of a naïve competitor suggests that co-evolution may play a role in resistance to allelopathy.
Although metabolomics and proteomics have been used to study responses of diatoms to various stressors, the current study represents the first instance of metabolites and proteins measured simultaneously to understand the effects of competition in an ecological setting. Allelopathic compounds appear to target multiple physiological pathways in sensitive competitors, demonstrating that chemical cues released by plankton have the potential to alter large scale ecosystem processes including primary production and nutrient cycling. Understanding the impacts of these allelopathic compounds on competing species is critical for modeling bloom formation, structuring and maintenance and global-scale nutrient cycling in the ocean.
Appeal to a popular (non-scientific) audience
The harmful algal bloom-forming dinoflagellate Karenia brevis is episodically abundant along several coastlines and their blooms are increasing in density and frequency. In addition to introducing the chemical ecology strategy of allelopathy to your general readership, this study will provide readers with a unique, yet simplified, glimpse into the metabolic responses of competitors when exposed to harmful chemicals released by a bloom-forming alga.
Integrating metabolomic and proteomic analyses, we identified how allelopathy impacts competitor metabolism and what biochemical strategies are undertaken by competitors to adapt to allelopathy. Effects on competitor physiology were reflected in the expressed metabolomes and proteomes of two diatom species, with the diatom that co-occurs with K. brevis blooms (A. glacialis) exhibiting more robust metabolism in response to K. brevis. The greater metabolic sensitivity of a naïve competitor suggests that co-evolution may play a role in resistance to allelopathy.
Although metabolomics and proteomics have been used to study responses of diatoms to various stressors, the current study represents the first instance of metabolites and proteins measured simultaneously to understand the effects of competition in an ecological setting. Allelopathic compounds appear to target multiple physiological pathways in sensitive competitors, demonstrating that chemical cues released by plankton have the potential to alter large scale ecosystem processes including primary production and nutrient cycling. Understanding the impacts of these allelopathic compounds on competing species is critical for modeling bloom formation, structuring and maintenance and global-scale nutrient cycling in the ocean.
Appeal to a popular (non-scientific) audience
The harmful algal bloom-forming dinoflagellate Karenia brevis is episodically abundant along several coastlines and their blooms are increasing in density and frequency. In addition to introducing the chemical ecology strategy of allelopathy to your general readership, this study will provide readers with a unique, yet simplified, glimpse into the metabolic responses of competitors when exposed to harmful chemicals released by a bloom-forming alga.
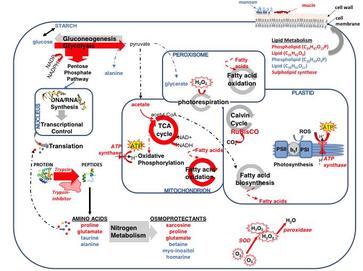
Figure : Network of cellular pathways, enzymes, and metabolites in the diatom Thalassiosira pseudonana impacted by exposure to Karenia brevis allelopathy, derived from NMR and MS metabolomics and proteomics. Pathways and metabolites up-regulated by allelopathy are indicated by red arrows and compound names, respectively. Blue arrows and compound names denote pathways and metabolites down-regulated by allelopathy. --manuscript in review